
This translocation is associated with triggering of morphological changes of nuclei, 13 although the mechanisms are not established. 12 Another characteristic event related to the nuclear changes during terminal differentiation is translocation of the N-terminal fragment of profilaggrin into the nucleus.

However, the same group recently reported that caspase-14-deficient mice are prone to development of parakeratosis (persistent presence of nuclei in the cornified layer) in conditions triggering epidermal hyperproliferation. 11 reported that although caspase-14 knockout mice showed defects in filaggrin (FLG) degradation, there was no effect on denucleation. 9, 10 It has been suggested that caspase-14 is not involved in apoptosis or inflammation, but participates in keratinocyte terminal differentiation.
ICAD ANTIBODY SKIN
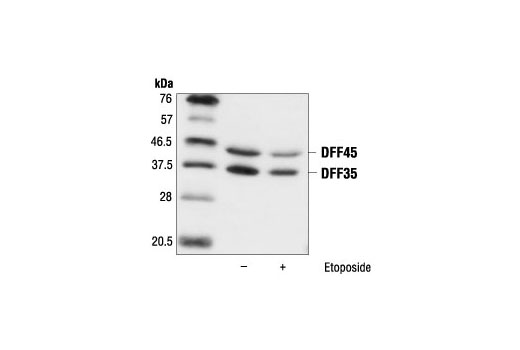
Unlike other caspase family members, caspase-14 shows restricted expression, being found only in epidermis, skin appendages and thymic Hassall’s bodies. However, proapoptotic caspases are not activated during terminal differentiation. 4, 5 Executioner caspases cause limited proteolysis of ICAD (inhibitor of caspase-activated DNase), releasing CAD (caspase-activated DNase) that is translocated into the nucleus, where it causes DNA degradation. 3 Caspases are cysteine proteases that regulate apoptosis and inflammation. 2 DNase 2, the predominant DNase on the mammalian skin surface, primarily targets exogenous DNA. However, DNase 1L2-deficient mice have a unique phenotype, with undigested nuclei being present only in hair shaft and nail plate. It has long been suggested that DNase 1L2 is responsible for denucleation in epidermis. 1 Although this is an important issue in connection with formation of the protective barrier of the skin, the mechanisms of DNA degradation during keratinocyte terminal differentiation remain unclear. Persistent presence of undigested nuclei in cornified cells (parakeratosis) is correlated with impaired barrier function, as shown by higher values of transepidermal water loss (TEWL), an indicator of barrier function. Collectively, our results indicate that at least two pathways are involved in the DNA degradation process during keratinocyte terminal differentiation. Immunohistochemical study revealed that both caspase-14 and mesotrypsin were markedly downregulated in parakeratotic areas of lesional skin from patients with atopic dermatitis and psoriasis. Knockdown of both proteases resulted in a significant increase of remnant nuclei in a skin equivalent model. Furthermore, caspase-14 caused limited proteolysis of ICAD, followed by accumulation of caspase-activated DNase (CAD) in TUNEL-positive nuclei. Mutation in the mesotrypsin-susceptible Arg-rich region between FLG-N and the first filaggrin domain abolished these changes. Interestingly, these cells became TUNEL positive. First, we demonstrated that epidermal mesotrypsin liberated a 55-kDa N-terminal fragment of profilaggrin (FLG-N) and FLG-N was translocated into the nucleus.

To elucidate the mechanisms involved, we focused on two characteristic events: nuclear translocation of N-terminal fragment of profilaggrin and caspase-14-dependent degradation of the inhibitor of caspase-activated DNase (ICAD). The purified CAD had DNase activity with a high specific activity.Loss of the nucleus is a critical step in keratinocyte terminal differentiation. The complex was treated with caspase 3, and CAD was purified to homogeneity. Recombinant CAD, as a complex with ICAD-L, was then produced in Sf9 cells. These results indicated that ICAD-L but not ICAD-S works as a specific chaperone for CAD, facilitating its correct folding during synthesis. Addition of ICAD-L but not ICAD-S to the assay mixture resulted in the synthesis of functional CAD. In vitro transcription and translation of CAD cDNA did not produce a functional protein. However, when CAD was co-expressed with ICAD-L and ICAD-S, it was recovered as a soluble protein complexed predominantly with ICAD-L. When CAD was expressed alone in Sf9 cells, it was found in insoluble fractions. CAD was predominantly associated with ICAD-L in these cell lines. Here, we report that mouse WR19L and human Jurkat T lymphoma cells express two alternative forms of ICAD, ICAD-L and ICAD-S, at similar levels. CAD is complexed with an inhibitor of CAD (ICAD) in non-apoptotic, growing cells.
Caspase-activated DNase (CAD) is responsible for the DNA fragmentation that occurs during apoptosis.


 0 kommentar(er)
0 kommentar(er)
